Answer:
0.322 mol.
Step-by-step explanation:
Hello there!
In this case, because we can understand the mole as the amount of substance of any species, we can say that one mole of any compounds equal the molar mass of the considered compound, it means that 1 mole of calcium phosphate has a mass of 310.18 g and therefore, the moles in 100 g of this salt turns out to be:
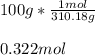
Best regards!