Answer: The percentage by mass of sulphur in
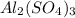 is 9.36%
is 9.36%
Step-by-step explanation:
Mass percent of an element is the ratio of mass of that element by the total mass expressed in terms of percentage.
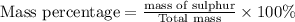
Given: mass of sulphur = 32 g/mol
mass of
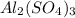 = 342 g/mol
= 342 g/mol
Putting in the values we get:
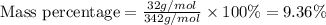
The percentage by mass of sulphur in
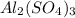 is 9.36%
is 9.36%