Answer:
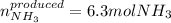
Step-by-step explanation:
Hello there!
In this case, according to the given chemical reaction, it is possible for us to use the 1:2 mole ratio of nitrogen to ammonia to calculate the moles of the latter that are produced when reacting 3.15 moles of the former with hydrogen as shown below:
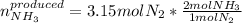
Thus, by solving the equation we obtain:
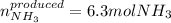
Best regards!