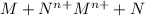Answer: a. Cathode
b. Galvanic cell
c. Anode
d. Electrolytic cell
e. half reaction
Step-by-step explanation:
Galvanic cell or Electrochemical cell is defined as a device which is used for the conversion of the chemical energy produced in a spontaneous redox reaction into the electrical energy.
Electrolytic cell is a device where electrical energy is used to drive a non spontaneous chemical reaction.
In the electrochemical cell, the oxidation occurs at an anode which is a negative electrode and the reduction occurs at the cathode which is a positive electrode. Thus the electrons are produced at anode and travel towards cathode.
The balanced two-half reactions will be:
Oxidation half reaction :
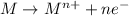
Reduction half reaction :
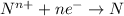
Thus the overall reaction will be: