Answer: The average atomic mass of nitrogen is 14.0066 amu
Step-by-step explanation:
Mass of isotope N-14 = 14.00307 amu
% abundance of isotope N-14= 99.64% =
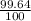
Mass of isotope N-13 = 15.0001 amu
% abundance of isotope N-13= (0.36)% =
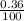
Formula used for average atomic mass of an element :
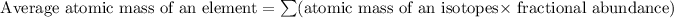
![A=\sum[(14.00307)* (99.64)/(100))+(15.0001)* (0.36)/(100)]]](https://img.qammunity.org/2022/formulas/chemistry/college/dnaacxi3rqi52tw263c9e8fjcgd3x1vvua.png)
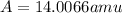
Therefore, the average atomic mass of nitrogen is 14.0066 amu