Answer:
T₂ = 478.93 K
Step-by-step explanation:
Given that,
Initial pressure, P₁ = 178 kPa
Initial temperature, T₁ = 37°C = 310 K
Final pressure, P₂ = 275 kPa
We need to find the new temp of the air in the tire, assuming the volume is constant. We know that,
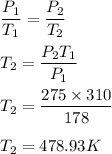
So, the new temperature is equal to 478.93 K.