Answer:
2.1 M
Step-by-step explanation:
The dilution equation is
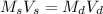 .
.
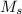 = the molarity of the sock solution
= the molarity of the sock solution
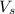 = the volume of the sock solution
= the volume of the sock solution
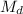 = the molarity of the diluted solution
= the molarity of the diluted solution
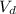 = the volume of the diluted solution
= the volume of the diluted solution
The stock solution would be what is doing the diluting, so "175 mL of a 3.00 M solution". So
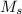 = 3.00 M. Then: Converting 175 mL to liters:
= 3.00 M. Then: Converting 175 mL to liters:
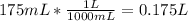 (This is
(This is
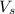 )
)
And converting 250 mL KCl to liters:
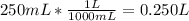 (This will be
(This will be
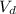 )
)
Then, we plug in our given into the dilution equation, resulting in:
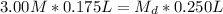 (divide both sides by 0.250 L, in order to get
(divide both sides by 0.250 L, in order to get
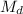 by itself)
by itself)
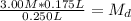
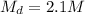
So, the molarity of 250 mL KCl made by diluting 175 mL of a 3.0 M solution would be 2.1 M (mol/L).
Hopefully this helped you understand the topic a little bit better. I just finished molarity and dilutions in Chemistry last week. Good luck!