Answer:
The scaling factor is 5.
Step-by-step explanation:
Hello there!
In this case, since the scaling factor is defined as the ratio of the molar mass of the molecular formula (complete) to the empirical formula (simplified), it is possible to compute it for the empirical formula of CH2O whose molar mass is 30 g/mol (12+2+16) as shown below:
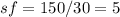
Therefore, we can also infer that the molecular formula would be:
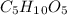
Best regards!