Answer:
the percent error is -13.5%
Step-by-step explanation:
Given the data in the question;
Exact atomic weight = 65.38 g/mol
For approx
increased mass = 0.3681 g
moles = 6.514 x 10⁻³
we know that; moles = mass / (Atomic mass )
so
Atomic mass_
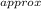 = mass / moles
= mass / moles
Atomic mass_
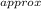 = 0.3681 / 6.514 x 10⁻³
= 0.3681 / 6.514 x 10⁻³
Atomic mass_
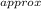 = 56.509
= 56.509
so approx atomic weight = 56.51 g/mol
we know that;
% error = ( [approx - exact] / exact ) × 100
we substitute
% error = ( [56.51 - 65.38] / 65.38 ) × 100
% error = (-8.87 / 65.38 ) × 100
% error = ( -0.1356684 ) × 100
% error = -13.5%
Therefore, the percent error is -13.5%