Given :
A compound is found to be made up of 3.21 g Carbon and 1.02 g Oxygen.
To Find :
The percent composition from this data.
Solution :
We know, percentage composition is given by :
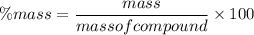
So, percentage composition of Carbon is :
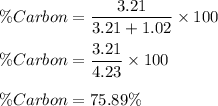
Since, compound is made up of Carbon and Oxygen.
So, %Oxygen is ( 100 - 75.89 )% = 24.11%
Hence, this is the required solution.