Answer:
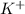 and
and
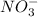
Step-by-step explanation:
The equation for the reaction is AgNO3(aq) + KCl(aq) ==> AgCl(s) + KNO3(aq)
With all the ions, it is
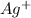 (aq) +
(aq) +
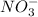 (aq) +
(aq) +
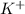 (aq) +
(aq) +
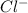 (aq) ==> AgCl(s) +
(aq) ==> AgCl(s) +
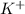 and
and
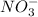 do not change, so they are the spectator ions and are removed
do not change, so they are the spectator ions and are removed
The ionic equation is:
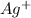 (aq) +
(aq) +
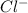 (aq) ==> AgCl(s)
(aq) ==> AgCl(s)