Answer:

Step-by-step explanation:
To convert from grams to moles, we must use the molar mass. This can be found on the Periodic Table. First, find the molar mass of iron and chlorine.
- Fe: 55.84 g/mol
- Cl: 35.45 g/mol
Check the formula. There is a subscript of 3 after Cl, so there are 3 atoms of chlorine in 1 molecule. Multiply iron's molar mass by 3, then add iron's molar mass.
- FeCl₃: 55.84 + 3(35.45) = 55.84+106.35=162.19 g/mol
Use this number as a ratio.
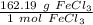
Multiply by the given number of grams.
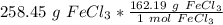
Flip the ratio so the grams of iron (III) chloride cancel.
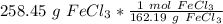
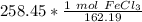
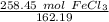
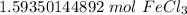
The original measurement of grams has 5 significant figures, so our answer must have the same. For the number we calculated, that is the ten thousandth place.
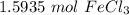
258.45 grams is approximately 1.5935 moles of iron (III) chloride.