Answer:
6.3 × 10–^4
Step-by-step explanation:
Hello there!
In this case, since the rate laws are written in terms of the rate constant and the concentration of the species contributing to the rate of reaction, as this one is second order in Xe and first order in F2, the rate law would be:
![r=k[Xe]^2[F_2]](https://img.qammunity.org/2022/formulas/chemistry/high-school/2qncvcc95efwvt8s5soer6pxc0i6ng6xfy.png)
Thus, by plugging in the rate constant and concentrations, we obtain:
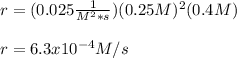
Best regards!