Answer:

Step-by-step explanation:
To convert from representative particles to moles, Avogadro's Number: 6.02*10²³, which tells us the number of particles (atoms, molecules, etc.) in 1 mole of a substance.
We can use it in a ratio.
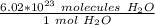
Multiply by the given number of molecules.
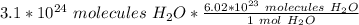
Flip the ratio so the molecules of water cancel out.
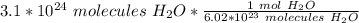
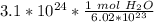
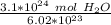
Divide.
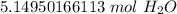
The original number of molecules has 2 significant figures: 3 and 1, so our answer must have the same. For the number we calculated, that is the tenth place. The 4 in the hundredth place tells us to leave the 1.
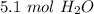
There are about 5.1 moles of water in 3.1*10²⁴ molecules of water.