Answer:
Compared to the initial Celsius temperature of the gas, the final Celsius temperature is greater by a factor of more than 2.
Step-by-step explanation:
Gay-Lussac's law states that the pressure of a fixed volume of a gas is directly proportional to its temperature. In other words, if the temperature increases, the pressure will increase and if the temperature decreases, the pressure will decrease.
In summary, Gay-Lussac's law is a law that says that when the amount of gas and volume are kept constant, the quotient that exists between the pressure and the temperature will always have the same value:
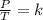
Being an initial state 1 and a final state 2, it is true:
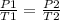
In this case:
Replacing:
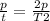
Solving:
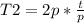
T2= 2*t
Compared to the initial Celsius temperature of the gas, the final Celsius temperature is greater by a factor of more than 2.