Answer: d)endothermic with a positive entropy change
Step-by-step explanation:
Endothermic reactions are those in which heat is absorbed by the system and exothermic reactions are those in which heat is released by the system.
Entropy is the measure of randomness or disorder of a system. If a system moves from an ordered arrangement to a disordered arrangement, the entropy is said to decrease and vice versa.
As temperature of water decreases on dissolution of
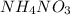 , it means heat has been absorbed by
, it means heat has been absorbed by
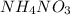 during dissolution and that heat is taken from water. Thus process is endothermic. As dissolution will lead in the formation of ions
during dissolution and that heat is taken from water. Thus process is endothermic. As dissolution will lead in the formation of ions
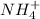 and
and
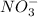 , randomness will increase and thus there is a positive entropy change.
, randomness will increase and thus there is a positive entropy change.