Answer:
a. changes with temperature.
Step-by-step explanation:
Hello there!
In this case, according to the thermodynamic definition of the equilibrium constant in terms of the Gibbs free energy of reaction and the temperature of the system:
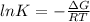
It is possible to figure out that the equilibrium constant varies as temperature does, not only on the aforementioned definition, but also in the Gibbs free energy as it is also temperature-dependent. Therefore, the appropriate answer is a. changes with temperature.
Best regards!