Answer: compressibility
Step-by-step explanation:
The ratio of the measured molar volume of a real gas to the measured molar volume of an ideal gas is known as compressibility.
Compressibility is defined by the symbol Z and is calculated by the formula :
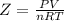
For a gas with ideal behavior, molar volume of an ideal gas is equal to the molar volume of a real gas so Z= 1.