Answer:
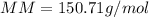
Step-by-step explanation:
Hello there!
In this case, since the molar masses of chemical compounds allow us to understand the mass they have per mole of substance, for tin oxide, we can see it has one tin atom and two oxygen atoms; thus, for the calculation of this molar mass we multiply the atomic masses by the number of atoms and them add the results up:
Best regards!