Answer:
0.4816 mol S
Step-by-step explanation:
I'm going to answer this question under the assumption that the question is how many moles of sulfur are present in 34.68 grams of disulfur pentoxide.
The molecular formula of disulfur pentoxide is S₂O₅ (di means two and penta means five).
Now we convert 34.68 g of S₂O₅ into moles, using its molar mass:
- 34.68 g ÷ 144 g/mol = 0.2408 moles S₂O₅
Then we convert moles of S₂O₅ into moles of S, given that there are two moles of S per S₂O₅ mol:
- 0.2408 moles S₂O₅ *
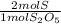 = 0.4816 mol S
= 0.4816 mol S