After performing a stoichiometry calculation using the provided moles of nitrogen and hydrogen, 0.580 moles of ammonia are produced after a complete reaction.
When nitrogen and hydrogen react to produce ammonia, the reaction follows a stoichiometric ratio according to the balanced chemical equation:
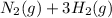 →
→
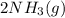
To solve for the moles of ammonia produced, we must first determine the limiting reactant. We are given 0.290 mol
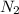 and 0.954 mol
and 0.954 mol
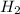 . The stoichiometric ratio of hydrogen to nitrogen is 3:1 from the balanced equation; thus, to fully react with 0.290 mol of nitrogen, we would need 0.290 mol x 3 = 0.870 mol of hydrogen.
. The stoichiometric ratio of hydrogen to nitrogen is 3:1 from the balanced equation; thus, to fully react with 0.290 mol of nitrogen, we would need 0.290 mol x 3 = 0.870 mol of hydrogen.
Since 0.954 mol of hydrogen is provided, which is more than needed, nitrogen is the limiting reactant.
Every 1 mol of
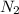 produces 2 mol of
produces 2 mol of
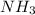 , so 0.290 mol of
, so 0.290 mol of
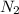 will produce 0.290 mol x 2 = 0.580 mol of
will produce 0.290 mol x 2 = 0.580 mol of
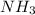 . Therefore, after the complete reaction, 0.580 moles of ammonia are produced.
. Therefore, after the complete reaction, 0.580 moles of ammonia are produced.