Answer: 4.5 moles
Step-by-step explanation:
To understand how to solve this problem, you must understand the ratios written in this chemical equation.
The equation shows that 4 moles of Al forms 2 moles Al₂O₃. This creates the ratio 2:4 or
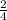
To solve, you can set the two ratios to each other and cross multiply.
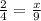
18 = 4x
x = 4.5 mol Al₂O₃
*both
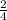 and
and
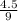 can be simplified as
can be simplified as
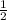 , which verifies your answer*
, which verifies your answer*