Answer:
mass = 44.2 g
Step-by-step explanation:
We are asked to calculate the mass of 1.3 mol of hydrogen sulfide, H₂S.
To do this, we can use the following formula:
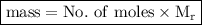 .
.
The
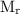 (relative molecular mass) of H₂S is:
(relative molecular mass) of H₂S is:
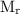 = (2 × 1) + 32
= (2 × 1) + 32
= 2 + 32
= 34
Therefore, using the formula above and substituting the data, we get:
mass = 1.3 × 34
= 44.2 g