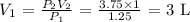Answer:
Step-by-step explanation:
Here, we want to calculate the initial volume of the container
Mathematically, we know that volume and pressure are inversely related. What this means is that as volume increases, pressure is expected to decrease and as pressure increases, volume is expected to decrease
A mathematical link between these two is as follows:
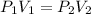
The above is according to Boyles' law.
The values with subscript 1 are the initial values, while the values with the subscript 2 are the final values
Thus:
V1 = ?
P1 = 1.25 atm
V2 = 1L
P2 = 3.75 atm
From the relation: