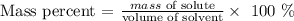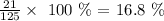Answer:
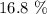
Step-by-step explanation:
Here, we want to get the mass percent of a NaCl with 125.0L of pure water
What we have to do here is to divide the mass of the solute by the volume of the solvent and multiply by 100%
Mathematically, we have this as: