Answer:
- Anode: Co3+ | Co2+
- Cathode: Ni | Ni2+
Step-by-step explanation:
The anode is where oxidation reaction occurs, and the cathode is where reduction reaction occurs.
From the table of reduction potencials, we find that:
- Co reaction:
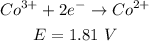
- Ni reaction:
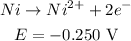
Now, to find out which one is the anode and which one is the cathode, it is necessary to compare the reduction potencials.
The reaction of Ni have negative potentials, so Ni will be the anode and Co will be the cathode.