Answer:
87.22grams
Explanations:
The chemical reaction between Nitrogen dioxide and water to produce nitric acid and nitrogen monoxide is expressed as:
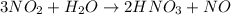
Determine the moles of nitrogen dioxide(NO2)
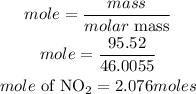
According to stoichiometric ratio, 3 moles of nitrogen dioxide produces 2moles of nitric acid, the moles of nitric acid required is expressed as;
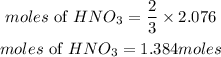
Determine the mass of nitric acid
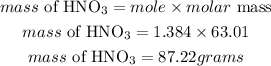
Hence the mass of nitric acid required is 87.22grams