To answer this question we have to use Charles' Law:
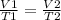
Before replacing for the given values, we have to convert the given temperatures to Kelvin:
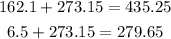
Now replace for the given values, V1 and V2 are the initial and final volumes respectively and T1 and T2 are the initial and final temperatures. Solve for V1 and find its value:
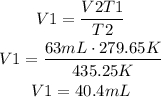
It means that the initial volume was approximately 40mL.
The correct answer is c. 40.