Answer:
0.0000253moles
Explanations:
The decomposition of NI3 is given as shown below;
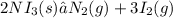
Given the following parameters
Mass of NI3 = 0.02 grams
Determine the moles of NI3
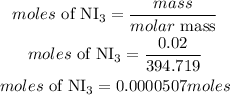
Acoording to stochiometry, 2 moles of NI3 preoduces 1 mole of nitrogen. The mole of nitrogen produced is;
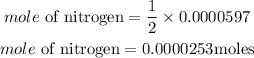
Hence the moles of nitrogen produced is 0.0000253moles