Answer:
10L
Explanations:
The formula for calculating the molarity of a solution is expressed as:
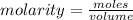
Given the following parameters
• Moles of salt = 5 moles
,
• Molarity of solution = 0.5M
Substitute the given parameters into the formula
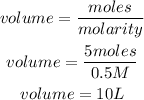
Hence the required volume of the solution is 10L