Given that the volume of gas kept at constant pressure varies inversely with the temperature T and when the temperature is 50 degrees, the volume is 20 cubic feet.
We have to find the volume of gas when the temperature is 100 degrees.
Since it is given that volume varies inversely with the temperature. It means
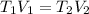
Substitute T1 = 50, V1 = 20, T2 = 100.
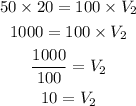
Thus, the volume of gas when the temperature is 100 degrees is 10 cubic feet.