Answer:
There are 4.34 moles of solute.
Step-by-step explanation:
The concentration of the solution of 7.0M, means that there are 7.0 moles of solute in 1000mL (1L) of solution.
In this case, we only have 620mL, so with the concentration and a mathematical rule of three we can calulate the moles of solute in 620mL:
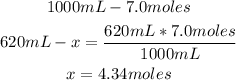
Finally, there are 4.34 moles of solute.