Answer
0.02547 moles of CO2 are present in 1.534x10²² molecules
Step-by-step explanation
1 mole of any substance contains 6.022 × 10²³ molecules.
So, x moles of CO₂ are present in 1.534x10²² molecules
x moles of CO₂ is equal to
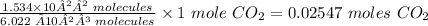
Thus, 0.02547 moles of CO2 are present in 1.534x10²² molecules