The first step to solve this question is to find the pOH of the solution, to do it, we have to use the following formula:
![pOH=-log[OH^-]](https://img.qammunity.org/2023/formulas/chemistry/college/vqo02fhb0jj8u1geaydn77je0d54m8v5tf.png)
Where [OH-] is the concentration of hydroxide ions.
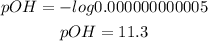
Now, we have to find the pH of the solution. Remember that the sum of pH and pOH is always 14, we can use this information to find the value of the pH:
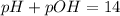
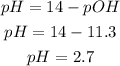
We can find the hydronium ion concentration using the following equation:
![\begin{gathered} pH=-log\lbrack^H^+] \\ 10^{-\frac{}{}pH}=\lbrack H^+] \\ 10^(-2.3)=\lbrack H^+] \\ \lbrack H^+]=0.005M \end{gathered}](https://img.qammunity.org/2023/formulas/chemistry/college/xylvmlec8skoiefhrt7i19rqovti6rerpy.png)
It means that the concentration of hydronium ions is 0.005M