Firstly we need to determine the oxidation numbers of each element in the compound to determine which is undergoing reduction and which is undergoing oxidation. The oxidizing agent is the substance in the redox reaction that gains an electron from the reducing agent.
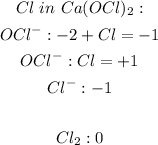
The Ca(OCl)2 is both undergoing reduction and oxidation therefore the oxidizing agent is indeed Ca(OCl)2.