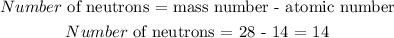Answer:
There are 14 protons
There are 14 neutrons
Step-by-step explanation:
Here, we want to get the number of protons and neutrons in the atom
Firstly, the atomic number is the same as the number of protons in the atom
That means, the atom has 14 protons
Mathematically, the sum of the number of protons and the number of neutrons equal the mass number
Thus: