The defect of Freundlich adsorption isotherm is that it fails at high pressure of the gas.
Langmuir derived the adsorption isotherm based on the theoretical considerations. It is generally applied to chemical adsorption.
It can be expressed as
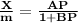
where,
X = mass of the gas adsorbed
M = mass of the adsorbent
P = equilibrium pressure