By using Avogadro's Law we have:
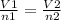
Where V is volume and n is moles, By substituting what have to find the unknown V2 gives:
11.0 L/ 7.70 moles = V2/ 2.97 moles
V2 = 11.0 L x 2.97 moles / 7.7 moles
V2 = 4.24 L. This is the volume of gas that would have escaped
New volume of the container is:
V= 11.0 L- 4.24 L
V= 6.76 L
Answer is 6.76 L