To find the mass percent of the solution, we have to divide the mass of solute by the mass of solution and multiply it by 100.
The mass of solution will be the sum of the mass of the solute and the mass of the solvent:
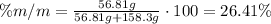
It means that the mass percent of NaCl in the solution is 26.41%