Answer:
There are 2 moles in 80.0g of NaOH.
Step-by-step explanation:
To calculate the moles of NaOH in 80.0g it is necessary to use the molar mass of NaOH:
- NaOH molar mass: 40g/mol
- Conversion from grams to moles:
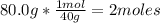
So, there are 2 moles in 80.0g of NaOH.