The first step is to determine if there is a limit reactant in the equation.
To do this, we have to use the ratios of the coefficients of the reactants to see how many moles of each of them must react with the given quantity of the other reactant.
For example, multiply 7.4 moles of aluminium by the ratio of copper (II) sulfate to aluminium:
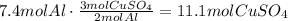
We need 11.1 moles of copper (II) sulfate to react with 7.4 moles of aluminium, but since we only have 5.4 moles of it, copper (II) sulfate is the limit reactant, so we have to base our calculations on this amount.
Use the amount of copper (II) sulfate and the ratio of the coefficients of copper and copper (II) sulfate:
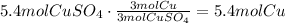
The answer is 5.4 moles of copper.