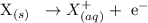ANSWER
Oxidation reaction
Step-by-step explanation
In an electrochemical cell, oxidation reaction occurs at the anode. This involves the loses of electrons, the electrons then flow through a wire to the cathode.
At the anode, oxidation reaction will take place