Answer
Option A = 103.2 mol KBr
Step-by-step explanation
Given:
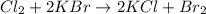
Moles of bromine = 51.62 moles
Required: Moles of KBr
Solution
Use the stoichiometry to calculate the the moles of KBr
The molar ration between Br and KBr is 1:2
Therefore the moles of KBr = 51.62 mol x 2 = 103.24 mol