Answer
48.95 grams of Cl₂
Step-by-step explanation
Given:
Mass of iron = 25.7 grams
What to find:
The mass of chlorine needed to use up 25.7 grams of iron.
Step-by-step solution:
The first step is to write a balanced chemical equation for the synthesis reaction.
2Fe + 3Cl₂ → 2FeCl₃
From the equation; 2 moles of Fe used up 3 moles of Cl₂
The molar mass of Fe = 55.845 g/mol and the molar mass of Cl₂ = 70.906 g/mol
This implies;
(2 mol x 55.845 g/mol) = 111.69 g of Fe used up (3 mol x 70.906 g/mol) = 212.745 g Cl₂
So, 25.7 g Fe will use up
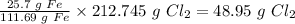
Therefore the mass of chlorine needed to use up 25.7 grams of iron is 48.95 grams