ANSWER
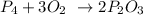
Explanation:
Given the reaction
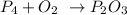
Looking at the above reaction, you will see that phosphorus is reacting with oxygen to produced phosphorus oxide.
To balance the equation, we will need one mole of phosphorus to react with 3 moles of oxygen to give 2 moles of phosphorus oxide