Answer
A) Paramagnetic
Step-by-step explanation
To determine the magnetic behavior of vanadium (V), you must write the electron configuration for V.
The atomic number of vanadium is 23 and its electron configuration is:
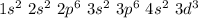
It has 3 unpaired d- electrons, so according to Hund's rule, Paramagnetic is the expected magnetic behavior of vanadium (V).
Hence, the correct answer is option A) Paramagnetic.