Since the reaction is
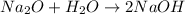
We would first need to balance it, but we can see that it was already balanced.
So, we can just apply the stoichiometry.
Assuming all the Na₂O react, we can just compair the coefficients of Na₂O and NaOH to calculate how much we need.
From the equation, we see that each 1 mol of Na₂O produce 2 moles of NaOH, so using rule of three we have:
Na₂O --- NaOH
x --- 1.57 mol
1 mol --- 2 mol
So we have the relation:
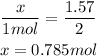
So, we need 0.785 mol of Na₂O.