Answer:
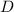
Step-by-step explanation:
Here, we want to write the equilibrium constant expression for the given reaction
To generate this, we have to get the concentration of the product raised to the coefficient in the balanced reaction, divided by the concentration of the reactants raised the same way
We do not factor for solid and liquid states when writing the equilibrium constant expression
We have the expression written as follows:
![K_(eq)\text{ = }\frac{1}{[H_2]\placeholder{⬚}^2[O_2]}](https://img.qammunity.org/2023/formulas/chemistry/college/ayosvdtl2pxxi5jjxo2zepvokbojlxf3uy.png)