Answer:
NO₂ is the limiting reactant.
The theoretical yield is 77.38 grams.
Explanations
Given the balanced chemical reaction as shown below:
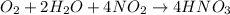
We are to find the theoretical yield of HNO₃ and the limiting reactant. To find the limiting reactant, we need to calculate the moles of each reactant and divide them by the total moles in the reaction.
For the moles of Oxygen:
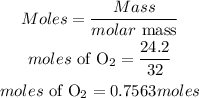
For the moles of water

Determine the moles of NO₂
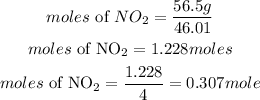
Since the compound with the least amount of moles is 0.307 moles, hence NO₂ is the limiting reactant.
Determine the mass of HNO₃(theoretical yield)
![\begin{gathered} Mass\text{ of HNO^^^^2083}=moles* molar\text{ mass} \\ Mass\text{ of HNO}_3=1.228moles*(63.01g)/(mol) \\ Mass\text{ of HNO}_3=77.38grams \\ \end{gathered}]()
Hence the mass of HNO₃ produced is 77.38 grams.