To calculate the number of moles we can use molarity, molarity relates the number of moles of solute to one liter of solution.
So we can replace molarity with:
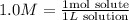
In this case, the solute is CuSO4. We replace in the equation:

So, the moles of CuSO4 are 0.108 moles